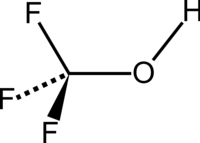
Trifluoromethanol
 | |
| Names | |
|---|---|
| Preferred IUPAC name Trifluoromethanol | |
| Other names Trifluoromethyl alcohol, perfluoromethanol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | CHF3O |
| Molar mass | 86.013 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.5±0.1 g/cm3 |
| Melting point | -110.64 |
| Boiling point | 22.4 °C (72.3 °F; 295.5 K) ±30.0°C |
| Hazards | |
| Flash point | 18.9 °C (66.0 °F; 292.0 K) ±15.6° |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Trifluoromethanol is a synthetic organic compound with the formula CHF
3O.[1] It is also referred to as perfluoromethanol or trifluoromethyl alcohol. The compound is the simplest perfluoroalcohol.[2] The substance is a colorless gas, which is unstable at room temperature.
Synthesis
Like all primary and secondary perfluoroalcohols, trifluoromethanol eliminates hydrogen fluoride in an endothermic reaction[3] and forms carbonyl fluoride.[4]
- CF
3OH ⇌ COF
2 + HF (I)
At temperatures in the range of -120 °C, trifluoromethanol can be prepared from trifluoromethyl hypochlorite and hydrogen chloride:
- CF
3OCl + HCl → CF
3OH + Cl
2 (II)
In this reaction, the recombination of a partially positively charged chlorine atom (in trifluoromethyl hypochlorite) with a partially negatively charged chlorine atom (in hydrogen chloride) is used as elemental chlorine. The undesired products, by-products chlorine, hydrogen chloride, and chlorotrifluoromethane, can be removed by evaporation at -110 °C. Trifluoromethanol has a melting point of -82 °C and a calculated boiling point of about -20 °C. The boiling point is thus about 85 K lower than that of methanol. This fact can be explained by the absence of intramolecular H—F bonds, which are also not visible in the infrared gas phase spectrum.
A simpler synthesis uses the reaction (I); an equilibrium can be shifted to the thermodynamically preferred trifluoromethanol at lower temperatures. If the synthesized trifluoromethanol is protonated by superacids, for example HSbF
6 (fluoroantimonic acid), the equilibrium can be further shifted to the left towards the desired product.
Similar to reaction (I), trifluoromethoxides (CF
3O−
) can be prepared from saline-type fluorides (e.g., NaF) and carbonyl fluoride. However, if the CF
3O−
ion is, for example, in an aqueous solution displaced by an acid, trifluoromethanol decomposes at the room temperature.
Occurrence in upper layers of atmosphere
While trifluoromethanol is unstable under normal conditions, it is generated in the stratosphere from CF−
3 and CF
3O−
radicals by reaction with OF+
and F−
radicals. In this case, decomposition of trifluoromethanol is negligible under the conditions prevailing in the atmosphere due to the high activation energy of the reaction. The expected lifetime of trifluoromethanol is several million years at altitudes below 40 km.[5][6]
See also
References
- ^ Kloeter, Gerhard; Seppelt, Konrad (January 1979). "Trifluoromethanol (CF3OH) and trifluoromethylamine (CF3NH2)". J. Am. Chem. Soc. 101 (2): 347–349. doi:10.1021/ja00496a012.
- ^ Seppelt, Konrad (May 1977). "Trifluoromethanol, CF3OH". Angewandte Chemie International Edition in English. 16 (5): 322–323. doi:10.1002/anie.197703221.
- ^ Schneider, W. F. (April 11, 1996). "Energetics and Mechanism of Decomposition of CF3OH". J. Phys. Chem. 100 (15): 6097–6103. doi:10.1021/jp952703m.
- ^ Seppelt, K. (1977). "Trifluormethanol, CF3OH. In: , ". Angew. Chem. (in German). 325 (89): 325. Bibcode:1977AngCh..89..325S. doi:10.1002/ange.19770890509.
- ^ Schneider, W. F. (January 1995). "Atmospheric Chemistry of CF3OH: Is Photolysis Important?". Environmental Science & Technology. 29 (1): 247–250. Bibcode:1995EnST...29..247S. doi:10.1021/es00001a031. PMID 22200226.
- ^ Wellington, T. J.; Schneider, W. F. (1994). "The Stratospheric Fate of CF3OH. In: Environmental Science & Technology 28/1994, S.". Environ. Sci. Technol. 28 (6): 1198–1200. doi:10.1021/es00055a036. PMID 22176252.
- v
- t
- e
| Alcohols found in alcoholic drinks |
|
|---|---|
| Medical alcohol |
|
| Toxic alcohols |
alcohols (1°)
| Methanol | |
|---|---|
| Ethanol |
|
| Butanol |
|
| Straight-chain saturated C1 — C9 |
|
| Straight-chain saturated C10 — C19 |
|
| Straight-chain saturated C20 — C29 |
|
| Straight-chain saturated C30 — C39 |
|
| Straight-chain saturated C40 — C49 |
|
alcohols (2°)
alcohols (3°)
- 2-Methyl-2-pentanol
- 2-Methylheptan-2-ol
- 2-Methylhexan-2-ol
- 3-Methyl-3-pentanol
- 3-Methyloctan-3-ol
- Diacetone alcohol
- Ethchlorvynol
- Methylpentynol
- Nonafluoro-tert-butyl alcohol
- tert-Amyl alcohol
- tert-Butyl alcohol
- Triphenylethanol
- Triphenylmethanol
| Monohydric alcohols |
|
|---|---|
| Dihydric alcohols | |
| Trihydric alcohols | |
| Polyhydric alcohols (sugar alcohols) |
|
fatty alcohols
unsaturated
fatty alcohols
- 3-Methyl-3-pentanol
- Erucyl alcohol
- Linolenyl alcohol
- Linoleyl alcohol
- Oleyl alcohol
- Palmitoleyl alcohol
- tert-Amyl alcohol
- tert-Butyl alcohol
| C1 — C7 |
|
|---|---|
| Deoxy sugar alcohols | |
| Cyclic sugar alcohols | |
| Glycylglycitols |
| Monoterpene alcohols | |
|---|---|
| Sesquiterpene alcohols | |
| Diterpene alcohols |
- 1,3-Difluoro-2-propanol
- 2,2,2-Trifluoroethanol
- 2-Fluoroethanol
- Nonafluoro-tert-butyl alcohol
- Trifluoromethanol
 Category
Category












