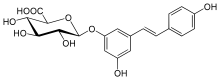
Trans-Resveratrol-3-O-glucuronide
 | |
| Names | |
|---|---|
| IUPAC name 3-Hydroxy-5-[(E)-2-(4-hydroxyphenyl)ethen-1-yl]phenyl β-D-glucopyranosiduronic acid | |
| Systematic IUPAC name (2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-{3-hydroxy-5-[(E)-2-(4-hydroxyphenyl)ethen-1-yl]phenoxy}oxane-2-carboxylic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C20H20O9 |
| Molar mass | 404.371 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
trans-Resveratrol-3-O-glucuronide is a metabolite of resveratrol[1] and trans-resveratrol-3-O-glucoside (piceid).[2][3]
References
- ^ Urpi-Sarda, M.; Zamora-Ros, R.; Lamuela-Raventos, R.; Cherubini, A.; Jauregui, O.; De La Torre, R.; Covas, M. I.; Estruch, R.; Jaeger, W.; Andres-Lacueva, C. (2006). "HPLC-Tandem Mass Spectrometric Method to Characterize Resveratrol Metabolism in Humans". Clinical Chemistry. 53 (2): 292–299. doi:10.1373/clinchem.2006.071936. PMID 17170057.
- ^ Zhou, M.; Chen, X.; Zhong, D. (2007). "Simultaneous determination of trans-resveratrol-3-O-glucoside and its two metabolites in rat plasma using liquid chromatography with ultraviolet detection". Journal of Chromatography B. 854 (1–2): 219–223. doi:10.1016/j.jchromb.2007.04.025. PMID 17500049.
- ^ Mikulski, D.; Molski, M. (2010). "Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside". European Journal of Medicinal Chemistry. 45 (6): 2366–2380. doi:10.1016/j.ejmech.2010.02.016. PMID 20199826.
- v
- t
- e
Hydroxystilbenes and their glycosides (monomeric forms)
- Pinosylvin
- 3,4′-Dihydroxystilbene
| |
| Combretastatins | |
|---|---|
| |||||
| |||||
 | This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e












