Caesium auride
 | |
 | |
 Solution of CsAu(left), pure CsAu(right) | |
| Names | |
|---|---|
| IUPAC name Caesium auride | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
PubChem CID |
|
InChI
| |
| |
| Properties | |
Chemical formula | AuCs |
| Molar mass | 329.872022 g·mol−1 |
| Appearance | Yellow crystals |
| Melting point | 580 °C (1,076 °F; 853 K)[1] |
Solubility in water | reacts violently |
| Structure | |
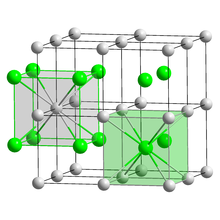
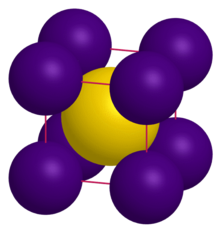
Crystal structure | CsCl |
Lattice constant | a = 4.24 Å[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Caesium auride is the inorganic compound with the formula CsAu. It is the Cs+ salt of the unusual Au− anion.[2]
Preparation and reactions
CsAu is obtained by heating a stoichiometric mixture of caesium and gold. The two metallic-yellow liquids react to give a transparent yellow product.[3] Despite being a compound of two metals, CsAu lacks metallic properties since it is a salt with localized charges; it instead behaves as a semiconductor with band gap 2.6 eV.[4]
The compound hydrolyzes readily, yielding caesium hydroxide, metallic gold, and hydrogen.[3]
- 2 CsAu + 2 H2O → 2 CsOH + 2 Au + H2
The solution in liquid ammonia is brown, and the ammonia adduct CsAu·NH3 is blue; the latter has ammonia molecules intercalated between layers of the CsAu crystal parallel to the (110) plane. Solutions undergo metathesis with tetramethylammonium loaded ion exchange resin to give tetramethylammonium auride.[3]
References
- ^ a b Kienast, Gerhard; Verma, Jitendra; Klemm, Wilhelm (June 1961). "Das Verhalten der Alkalimetalle zu Kupfer, Silber und Gold". Zeitschrift für anorganische und allgemeine Chemie (in German). 310 (3): 143–169. doi:10.1002/zaac.19613100304.
- ^ Peer, William J.; Lagowski, J. J. (1978). "Metal-Ammonia Solutions. 11. Au−, a Solvated Transition Metal Anion". J. Am. Chem. Soc. 100: 6260–6261. doi:10.1021/ja00487a064.
- ^ a b c Jansen, Martin (2005-11-30). "Effects of relativistic motion of electrons on the chemistry of gold and platinum". Solid State Sciences. 7 (12): 1464–1474. Bibcode:2005SSSci...7.1464J. doi:10.1016/j.solidstatesciences.2005.06.015.
- ^ Norrby, Lars J. (February 1991). "Why is mercury liquid? Or, why do relativistic effects not get into chemistry textbooks?". Journal of Chemical Education. 68 (2): 110. Bibcode:1991JChEd..68..110N. doi:10.1021/ED068P110.
Further reading
- Jansen, Martin (2008). "The chemistry of gold as an anion". Chemical Society Reviews. 37 (9): 1826–1835. doi:10.1039/B708844M. PMID 18762832.—includes photograph of the compound.
- v
- t
- e
- CsAu
- RbAu
- SiAu4
- (CH3)4NAu
- AuBr
- AuCl
- AuF
- AuI
- AuOH
- Au2S
- AuCN / KAu(CN)2
- Na3Au(S2O3)2
| Organogold(I) compounds |
|---|
- AuXe4(Sb2F11)2
- Au2(SO4)2
- Au4Cl8
- AuF3
- AuCl3
- AuBr3
- AuI3
- Au2O3
- Au(OH)3
- Au2S3
- AuPO4
- Au(C2H3O2)3
| Aurates(III) |
|---|
- AuF5
- AuF5·F2
- Au2(C2O4)5
- AuF
6 (predicted)












